Now Reading: Primer on the Uniform Code for Medical Device Marketing Practices
-
01
Primer on the Uniform Code for Medical Device Marketing Practices
Primer on the Uniform Code for Medical Device Marketing Practices
The Department of Pharmaceuticals recently released the draft Uniform Code of Medical Devices Marketing Practices (“UCMDMP”) on March 16, 2022 to regulate the marketing of medical devices by companies in India. It is a forward-looking document which will provide much needed clarity to the medical devices industry.
At present, medical devices companies may voluntarily adopt and follow the Uniform Code for Pharmaceutical Marketing Practices (“UCPMP”), which is primarily applicable to the pharmaceutical industry. The Department of Pharmaceuticals, acknowledging that the medical devices industry is a separate constituent of the healthcare system, has now framed the UCMDMP.
The UCMDMP has adopted a wide definition of health care professional (“HCP”) which includes any person or entity that is authorized to provide healthcare services or items to patients, or is involved in the decision to purchase, prescribe, order, use or recommend a medical device in India. This includes individual medical and healthcare practitioners, clinical establishments and pharmacies, and the administrative personnel at such establishments, but excludes HCPs who are bona fide employees of the company, while acting in that capacity.
PROMOTIONAL MATERIALS
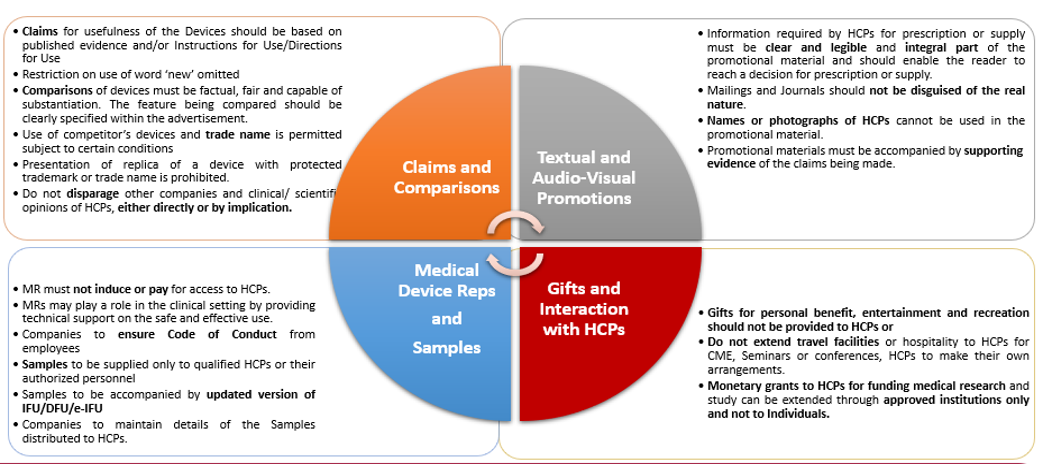
Promotional material has been defined as materials used by a medical devices company for the commercial promotion of its products and services either to an HCP or to a patient directly. The UCMDMP is medium-agnostic and applies to all marketing materials and advertisements of the company, but specifically excludes educational materials such as surgical technique portrayal, disease awareness material, research/ clinical material, evaluation reports or information/ documents that are mandated by regulatory provisions.
Follow the below chart to ensure all promotional materials are UCMDMP-compliant:
INDUSTRY-SPECIFIC FEATURES OF THE UCMDMP
The UCMDMP has done away with the restriction on the use of the word “new” for products that have been in the market for over a year, which is present under the UCPMP.
Medical devices must not be promoted prior to obtaining the requisite registrations and licenses for sale under the Medical Device Rules, 2017. The promotional material should be in consonance with the documents submitted for obtaining these registrations and licenses, and the claims for usefulness must be consistent with the Directions for Use that accompany the device.
Comparative Advertisements
Prior consent from a competitor need not be obtained for using their brand name as long as the feature of the competitor’s product that is the subject of comparison is clearly specified within the advertisement.
While the UCPMP outrightly prohibits the direct or implied disparagement of competitor’s products, services or promotions, the UCMDMP stipulates that any discussion and substantiation in this regard shall be based on available and published evidence.
Role of Medical Devices Representatives
The UCMDMP envisages a more involved role for medical device representatives in a clinical setting than medical representatives under the UCPMP. Medical device representatives, which includes clinical specialists in addition to sales representatives, are expected to provide technical support to HCPs on the safe and effective use of the devices, which may include explaining the unique setting and technical control functions, making recommendations, or assisting the HCP in clinical/operating rooms to ensure that the appropriate range of devices and accessories are available for a procedure.
Training of HCPS by Medical Devices Companies
Companies are expected to develop training sessions on the effective use of devices and their use in procedures, which will be a requirement for an HCP to get access to the product. These sessions may be delivered either by the medical device representatives of the company, or by engaging HCPs.
Allows for more collaboration with HCPs
The UCMDMP permits medical devices companies to collaborate with HCPs for activities such as consulting services, clinical studies and research, participation in company-conducted training and continued medical education sessions.
While the UCPMP permits companies to fund only medical research and studies at approved institutions, the UCMDMP also permits companies to provide educational grants to medical training institutions to support their legitimate scientific, educational, and training programs.
Permits certain gifts to HCPs
The UCMDMP contains most of the same restrictions that the UCPMP has placed on pharmaceutical companies, with a few relaxations. Medical devices companies may provide modest items to HCPs such as product manuals, anatomical models, e-books, subscriptions to online portals that provide medical content, etc. HCPs may occasionally also be provided with brand recall items having value less than one thousand rupees (Rs. 1000). These relaxations come with the obvious caveat that the gifts cannot be provided as incentive to prescribe the company’s products.
The UCMDMP, like the UCPMP, is proposed to be a voluntary code that companies may elect to adopt, though the government has specified that they may make it a statutory code if it is not effectively implemented. The draft UCMDMP is open for public consultation, and comments may be submitted to the Department of Pharmaceuticals till April 15, 2022.
| AUTHORS
Nusrat Hassan, Co-Managing Partner, Link Legal Pradnesh Warke, Associate Partner, Link Legal |
DISCLAIMER:
The contents of this article are for general information and discussion only and is not intended for any solicitation of work. This article should not be relied upon as a legal advice or opinion.